How to get the relative volatility
Relative volatility is defined as follows:
††††††††††††††††††††††††††††††††††††††††††††††††††††† 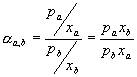 .......................................................... (1)
.......................................................... (1)
where †††† pa
is the partial pressure
†† xa
is the mole fraction in the liquid phase
Now bring in Raoultís law
††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† 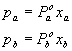 .................................................................. (2)
.................................................................. (2)
where††††† pa
is the partial pressure
†† Pao
is the vapour pressure
Combine Raoultís law with the definition for
relative volatility to get:
††††††††††††††††††††††††††††††††††††††††††††††††††††† ![]() .......................................................... (3)
.......................................................... (3)
This means that the relative volatility is the
same as the relative vapour pressure or ratio of vapour pressures.† If we know the vapour pressures we can
determine a.†
This is very useful.† We can get
vapour pressures at fixed temperatures from books such as Perryís Chemical
Engineers Handbook or the CRC Handbook of Chemistry and Physics.† The temperature range weíre interested in is
from the boiling point of one component to that of the other.† Relative volatility changes with temperature
but one value only is required for the next section.† That value is normally decided by averaging the relative volatilities
at the two limits of the temperature range.
How to determine vapour and liquid mole fractions
Next, the mole fractions in the vapour and
liquid phases can be determined from the relative volatility.
According to Daltonís law
†††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† ![]() .................................................................. (4)
.................................................................. (4)
where††††† PT
is the total pressure
†† ya
is the mole fraction in the vapour phase
Combine Daltonís law with the relative
volatility to give:
†††††††††††††††††††††††††††††††††††††††††††††††† ![]() ..................................................... (5)
..................................................... (5)
This is an expression that contains a, which we know from above, and the vapour and
liquid mole fractions.† Pick one, e.g.
the liquid mole fraction and we can determine the vapour mole fraction.† So we have the equation we want.† It could be rearranged, however, into a more
useful form.† See below:
†††††††††††††††††††††††††††††††††††††††††††††††† 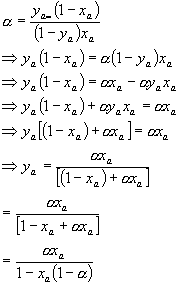 ..................................................... (6)
..................................................... (6)
Now, for different values of xa we
can determine ya.† Use an
average value for a (i.e. average of a at the higher and lower temperature limits).
Mole fractions using Raoult and Daltonís laws
Daltonís law and Raoultís law can be easily
combined to give an expression for ya.†
††††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† ![]() .................................................................. (7)
.................................................................. (7)
With this equation we can determine the vapour
phase mole fraction if we know the liquid phase mole fraction.† To get this we start with the fact that for
a binary mixture the total pressure is equal to the sum of the partial
pressures of the two components, i.e.
††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† ![]() ................................................................ (8)
................................................................ (8)
Substitute the expression for partial pressure
from Raoultís law into (8) to get:
††††††††††††††††††††††††††††††††††††††††††††††††† 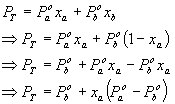 ..................................................... (9)
..................................................... (9)
Rearrange to give an expression in terms of xa
as follows:
††††††††††††††††††††††††††††††††††††††††††††††††††††††††††† ![]() ............................................................... (9)
............................................................... (9)
Now we can determine xa using the
vapour pressures and total pressure and from that we can use (7) to give ya.