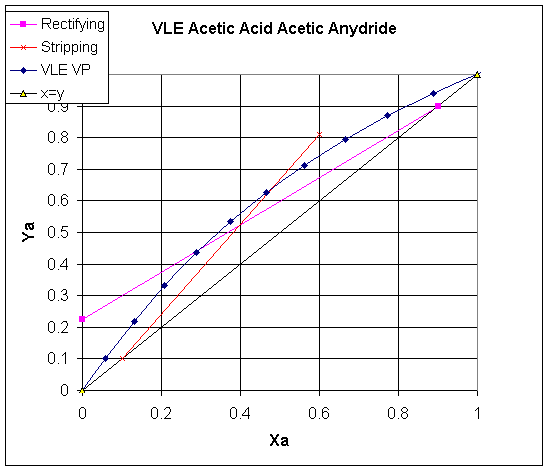
Solution to IA1 Acetic Acid Acetic Anhydride problem
(Statement
of problem: A mixture of Acetic Acid and Acetic Anydride containing 40 mol %
Acetic Acid is to be separated by distillation.† The top product is to be 90 mol % Acetic Acid and the bottom product
10 mol % Acetic Acid.† The feed flowrate
is 100 kmol/hr and enters the column as liquid at itís boiling point.
The
feed is heated to its boiling point.†
The vapour is condensed but not cooled and some is returned at a reflux
ratio of 3 kmol/kmol product.
Determine the operating lines for the
rectifying and stripping sections and draw them on the equilibrium curve.)
How
do we do this?
Start
with the rectifying line Ė it is the simplest to determine because we know the
reflux ratio.† The definition of this
line is:
![]()
In this case R = 3 and the mole fraction of a
(acetic acid) in the distillate (xd) is 0.9 so the equation becomes
![]()
Half way there.† All we need now is the equation of the stripping line.† To write this we need vapour and liquid
flowrates in the stripping section and the bottoms flowrate.† To get these we must do a mass balance.† We can write a mass balance on the whole
column for each component:
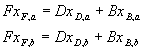
Again, a is acetic acid, b is acetic anhydride
(MVC first). We know the mole fractions, they are:
|
|
Feed |
Bottoms |
Distillate |
|
Acetic
acid |
0.4 |
0.1 |
0.9 |
|
Acetic
anhydride |
0.6 |
0.9 |
0.1 |
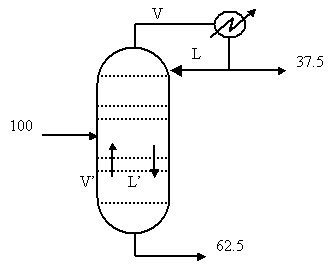
The flowrate of the feed, F, is 100
kmol/hr.† Equations above become:
![]()
Two equations for two unknowns can be solved to
give D = 37.5 kmol/hr and B = 62.5 kmol/hr.†
Draw a schematic of the column, add these numbers to it and see what
flowrates are left.
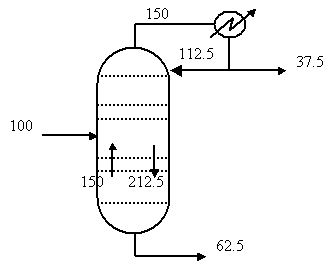
We must next determine the internal flowrates
in the column of vapour and liquid.
The reflux ratio is defined as R = L/D.† In this case R = 3 and D = 37.5 kmol/hr. †Therefore, L = 112.5 kmol hr.
The vapour flow of the top of the column is
condensed to give the L and D flowrates so V = L + D = 150 kmol hr.
The feed is all liquid at boiling point, i.e.
no vapour, only liquid but this liquid does not have to be heated to boiling
point, it is already at it.† This means
that the feed only contributes to the liquid flowrate down the column and not
to the vapour flowrate up the column.†
Therefore the vapour flowrate is the same in both the stripping and
rectifying sections, i.e. Ví = V = 150 kmol/hr.
The liquid flowrate in the stripping is the sum
of the feed and the rectifying flow, i.e. Lí = L + F = 212.5 kmol/hr.
Add these to the schematic:
Now, we can write the equation for the
stripping line:

All that is left is to plot these on the VLE
curve.